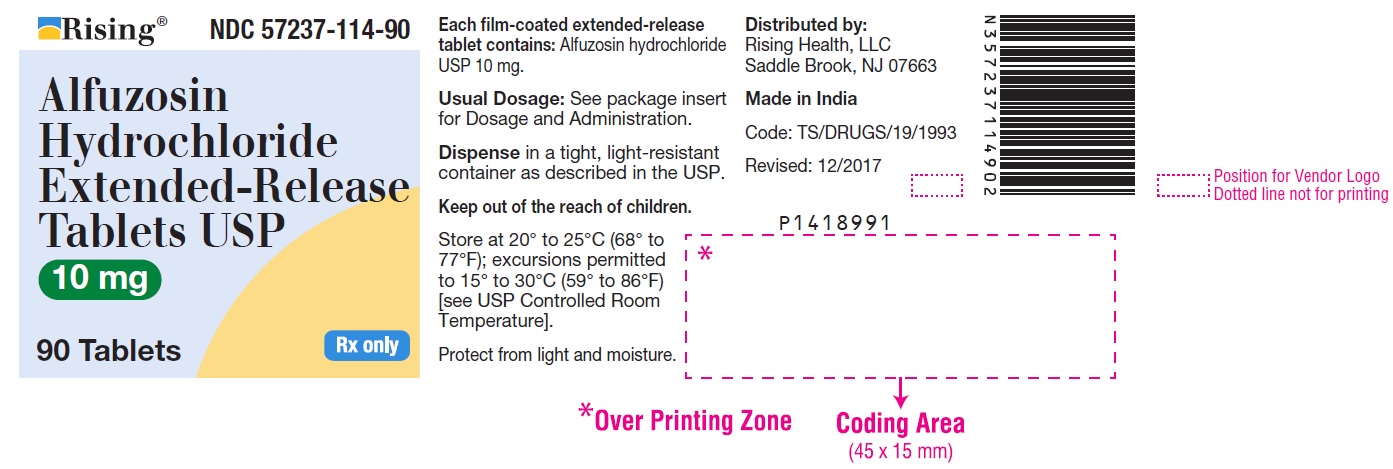
NDC Code(s) : 57237-114-90, 57237-114-01, 57237-114-05
Packager : Rising Pharma Holdings, Inc.
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Alfuzosin Hydrochloride Alfuzosin Hydrochloride TABLET, FILM COATED, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Rising Pharma Holdings, Inc.(116880195) |
| REGISTRANT - Aurobindo Pharma Limited(650082092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aurobindo Pharma Limited | 918917642 | ANALYSIS(57237-114), MANUFACTURE(57237-114) | |
PRINCIPAL DISPLAY PANEL
Rising® NDC 57237-114-90
Alfuzosin
Hydrochloride
Extended-Release
T
ablets USP
10 mg
90 Tablets Rx only