NDC Code(s) : 0121-1781-05, 0121-1781-00
Packager : PAI Holdings, LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Childrens AcetaminophenAcetaminophen SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - PAI Holdings, LLC(044940096) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| PAI Holdings, LLC dba Pharmaceutical Associates, Inc. and dba PAI Pharma | 097630693 | manufacture(0121-1781) | |
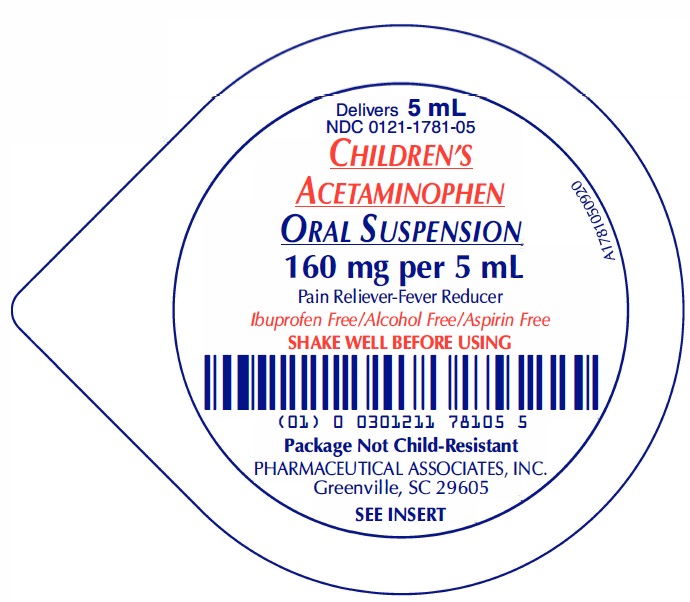
PRINCIPAL DISPLAY PANEL
Delivers 5 mL
NDC 0121-1781-05
CHILDREN'S ACETAMINOPHEN ORAL SUSPENSION
160 mg per 5 mL
Ibuprofen Free/ Alcohol Free / Aspirin Free
SHAKE WELL BEFORE USING
Package Not Child-Resistant
PHARMACEUTICAL ASSOCIATES, INC.
GREENVILLE, SC 29605
SEE INSERT