



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
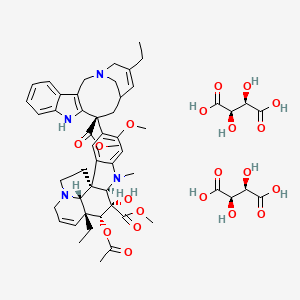

1. 5' Nor Anhydrovinblastine
2. 5'-nor-anhydrovinblastine
3. Kw 2307
4. Kw-2307
5. Kw2307
6. Navelbine
7. Vinorelbine
8. Vinorelbine Tartrate
1. Vinorelbine Tartrate
2. 125317-39-7
3. Kw 2307
4. Navelbine (tn)
5. Vinorelbine Ditartrate (jan)
6. Vinorelbine Bitartarate
7. Vinorelbine Tartrate (usp)
8. Schembl4334811
9. Schembl16766511
10. Amy22244
11. S4269
12. Akos037748842
13. Ccg-270632
14. As-75352
15. D01935
16. Bis((2r,3r)-2,3-dihydroxybutanedioic Acid); Methyl (1r,9r,10s,11r,12r,19r)-11-(acetyloxy)-12-ethyl-4-[(12s)-16-ethyl-12-(methoxycarbonyl)-1,10-diazatetracyclo[12.3.1.0(3),(1)(1).0?,?]octadeca-3(11),4,6,8,15-pentaen-12-yl]-10-hydroxy-5-methoxy-8-methyl-8,16-diazapentacyclo[10.6.1.0(1),?.0(2),?.0(1)?,(1)?]nonadeca-2(7),3,5,13-tetraene-10-carboxylate
| Molecular Weight | 1079.1 g/mol |
|---|---|
| Molecular Formula | C53H66N4O20 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 23 |
| Rotatable Bond Count | 16 |
| Exact Mass | 1078.42704051 g/mol |
| Monoisotopic Mass | 1078.42704051 g/mol |
| Topological Polar Surface Area | 364 Ų |
| Heavy Atom Count | 77 |
| Formal Charge | 0 |
| Complexity | 1820 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 11 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Vinorelbine tartrate |
| Drug Label | Vinorelbine Tartrate Injection is for intravenous administration. Each vial contains vinorelbine tartrate equivalent to 10mg (1-mL vial) or 50 mg (5-mL vial) vinorelbine in Water for Injection, USP. No preservatives or other additives are present.... |
| Active Ingredient | Vinorelbine tartrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 10mg base/ml |
| Market Status | Prescription |
| Company | Bedford; Hospira; Jiangsu Hansoh Pharm; Teva Pharms Usa; Actavis Elizabeth; Fresenius Kabi Usa; Hikma Maple; Ebewe Pharma; Dr Reddys Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Vinorelbine tartrate |
| Drug Label | Vinorelbine Tartrate Injection is for intravenous administration. Each vial contains vinorelbine tartrate equivalent to 10mg (1-mL vial) or 50 mg (5-mL vial) vinorelbine in Water for Injection, USP. No preservatives or other additives are present.... |
| Active Ingredient | Vinorelbine tartrate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 10mg base/ml |
| Market Status | Prescription |
| Company | Bedford; Hospira; Jiangsu Hansoh Pharm; Teva Pharms Usa; Actavis Elizabeth; Fresenius Kabi Usa; Hikma Maple; Ebewe Pharma; Dr Reddys Labs |
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)


