



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
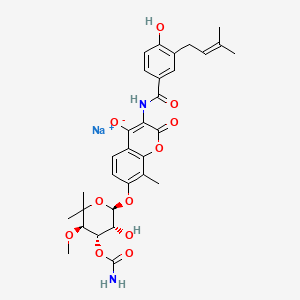

1. Calcium, Novobiocin
2. Crystallinic Acid
3. Monosodium Salt Novobiocin
4. Novobiocin
5. Novobiocin Calcium
6. Novobiocin, Monosodium Salt
7. Sodium, Novobiocin
8. Streptonivicin
1. Albamycin
2. Sodium Novobiocin
3. Cathomycin
4. Albadry
5. Novobiocin, Monosodium Salt
6. Novobiocin Monosodium Salt
7. Q9s9nq5yiy
8. 1476-53-5
9. Cathomycin Sodium
10. Chebi:31924
11. Nsc-2382
12. Vulcamycin
13. Drygard/biodry
14. Sodium Albamycin
15. Dsstox_cid_3386
16. Monosodium Novobiocin
17. Novobiocin Monosodium
18. Dsstox_rid_77008
19. Dsstox_gsid_23386
20. Cardelmycin Sodium Salt
21. Pa 93 Na Salt
22. Streptonivicin Sodium Salt
23. Inabiocin
24. Novobiocin, Sodium Deriv.
25. Albamycinsodium
26. Novobiocin Natrium
27. Albamycin Capsules
28. Novobiocina Bomaca
29. Component Of Albamycin Capsules
30. Albamycin (capsule)
31. Antibiotic From Streptomyces Spheroides
32. Novobiocin Sodium [usp]
33. Unii-q9s9nq5yiy
34. Novobiocin Sodium Salt (van)
35. U 6591
36. U-6591
37. Albamycin (tn)
38. Nsc 2382
39. Einecs 216-023-6
40. S2492_selleck
41. Ncgc00017033-01
42. Cas-1476-53-5
43. Novobiocin Sodium (jan/usp)
44. Spectrum1500444
45. Novobiocin Sodium [jan]
46. Chembl3183453
47. Dtxsid9023386
48. Hms500m22
49. Novobiocin Sodium [vandf]
50. Novobiocin Sodium [mart.]
51. Hms1920d04
52. Hms2091l04
53. Novobiocin Sodium [who-dd]
54. Tox21_110747
55. Ccg-40336
56. Akos030503807
57. Novobiocin Sodium [green Book]
58. Tox21_110747_1
59. Novobiocin Sodium [usp Impurity]
60. Ncgc00178810-03
61. Novobiocin Sodium [usp Monograph]
62. Novobiocin Sodium, Albamycin, Cathomycin
63. D01209
64. N-9100
65. Novobiocin Sodium, Meets Usp Testing Specifications
66. Q27114724
67. Novobiocin Sodium Salt, Antibiotic For Culture Media Use Only
68. 7-(4-(carbamoyloxy)tetrahydro-3-hydroxy-5-methoxy-6,6-dimethylpyran-2-yloxy)-4-hydroxy-3-(4-hydroxy-3-(4-hydroxy-3-(3-methyl-2-butenyl)benzamido)-8-methylcoumarin Monosodium Salt
69. 7-[(2r,3r,4s,5r)-4-carbamoyloxy-3-hydroxy-5-methoxy-6,6-dimethyl-tetrahydropyran-2-yl]oxy-3-[[4-hydroxy-3-(3-methylbut-2-enyl)benzoyl]amino]-8-methyl-2-oxo-chromen-4-olate
70. Benzamide, N-(7-((3-o-(aminocarbonyl)-6-deoxy-5-c-methyl-4-o-methyl-.alpha.-l-lyxo-hexopyranosyl)oxy)-4-hydroxy-8-methyl-2-oxo-2h-1-benzopyran-3-yl)-4-hydroxy-3-(3-methyl-2-buten-1-yl)-, Sodium Salt (1:1)
71. Benzamide, N-(7-((3-o-(aminocarbonyl)-6-deoxy-5-c-methyl-4-o-methyl-.beta.-l-lyxo-hexopyranosyl)oxy)-4-hydroxy-8-methyl-2-oxo-2h-1-benzopyran-3-yl)-4-hydroxy-3-(3-methyl-2-butenyl-, Monosodium Salt
72. Benzamide, N-(7-((3-o-(aminocarbonyl)-6-deoxy-5-c-methyl-4-o-methyl-beta-l-lyxo-hexopyranosyl)oxy)-4-hydroxy-8-methyl-2-oxo-2h-1-benzopyran-3-yl)-4-hydroxy-3-(3-methyl-2-butenyl)-, Monosodium Salt
73. Benzamide, N-(7-((3-o-(aminocarbonyl)-6-deoxy-5-c-methyl-4-o-methyl-beta-l-lyxo-hexopyranosyl)oxy)-4-hydroxy-8-methyl-2-oxo-2h-1-benzopyran-3-yl)-4-hydroxy-3-(3-methyl-2-butenyl-, Monosodium Salt
74. Sodium 7-(((2r,3r,4s,5r)-4-(carbamoyloxy)-3-hydroxy-5-methoxy-6,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-3-(4-hydroxy-3-(3-methylbut-2-en-1-yl)benzamido)-8-methyl-2-oxo-2h-chromen-4-olate
75. Sodium;7-[(2r,3r,4s,5r)-4-carbamoyloxy-3-hydroxy-5-methoxy-6,6-dimethyloxan-2-yl]oxy-3-[[4-hydroxy-3-(3-methylbut-2-enyl)benzoyl]amino]-8-methyl-2-oxochromen-4-olate
| Molecular Weight | 634.6 g/mol |
|---|---|
| Molecular Formula | C31H35N2NaO11 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 9 |
| Exact Mass | 634.21385422 g/mol |
| Monoisotopic Mass | 634.21385422 g/mol |
| Topological Polar Surface Area | 199 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 1160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)


