Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
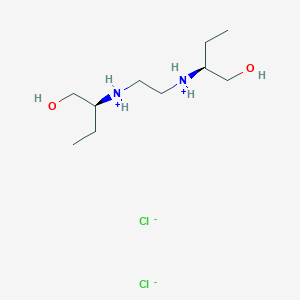
Also known as: Ethambutol hydrochloride, Ethambutol dihydrochloride, (+)-(s,s)-ethambutol dihydrochloride, (+)-2,2'-(ethylenediimino)di-1-butanol dihydrochloride, (+)-n,n'-bis(1-(hydroxymethyl)propyl)ethylenediamine dihydrochloride, (2s,2'e)-2,2'-(ethane-1,2-diyldiimino)dibutan-1-ol dihydrochloride
Molecular Formula
C10H26Cl2N2O2
Molecular Weight
277.23 g/mol
InChI Key
AUAHHJJRFHRVPV-BZDVOYDHSA-N
An antitubercular agent that inhibits the transfer of mycolic acids into the cell wall of the tubercle bacillus. It may also inhibit the synthesis of spermidine in mycobacteria. The action is usually bactericidal,...
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
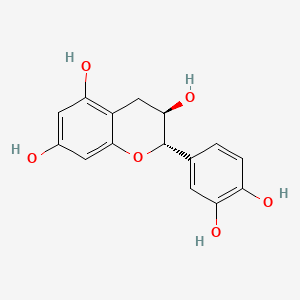
Also known as: 18829-70-4, Catechin l-form, (-)-catechol, (2s,3r)-2-(3,4-dihydroxyphenyl)chroman-3,5,7-triol, Catechin, alpha, (2s,3r)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2h-chromene-3,5,7-triol
Molecular Formula
C15H14O6
Molecular Weight
290.27 g/mol
InChI Key
PFTAWBLQPZVEMU-HIFRSBDPSA-N
FDA UNII
FHB0GX3D44
catechin is a natural product found in Smilax corbularia, Cinnamomum kotoense, and other organisms with data available.
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
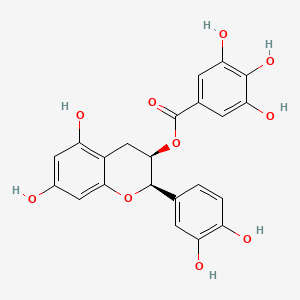
Also known as: 1257-08-5, Epicatechin gallate, (-)-epicatechin-3-o-gallate, (-)-epicatechingallate, (-)-epicatechin-3-gallate, L-epicatechin gallate
Molecular Formula
C22H18O10
Molecular Weight
442.4 g/mol
InChI Key
LSHVYAFMTMFKBA-TZIWHRDSSA-N
FDA UNII
92587OVD8Z
epicatechin gallate is a natural product found in Scurrula atropurpurea, Acacia omalophylla, and other organisms with data available.
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
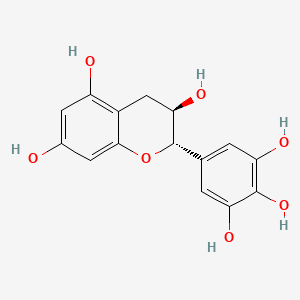
Also known as: 3371-27-5, (2s,3r)-2-(3,4,5-trihydroxyphenyl)chroman-3,5,7-triol, (2s,3r)-gallocatechin, Ent-gallocatechin, Chebi:71225, (2s,3r)-2-(3,4,5-trihydroxyphenyl)chromane-3,5,7-triol
Molecular Formula
C15H14O7
Molecular Weight
306.27 g/mol
InChI Key
XMOCLSLCDHWDHP-DOMZBBRYSA-N
(-)-gallocatechin is a natural product found in Senegalia catechu, Ziziphus mauritiana, and other organisms with data available.
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
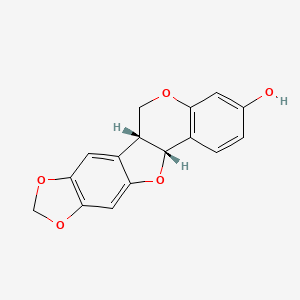
Also known as: (-)-maackiain, Inermin, 2035-15-6, Inermine, L-maackiain, (+/-)-maackiain
Molecular Formula
C16H12O5
Molecular Weight
284.26 g/mol
InChI Key
HUKSJTUUSUGIDC-ZBEGNZNMSA-N
FDA UNII
TF360D25IJ
(-)-maackiain is a natural product found in Cicer chorassanicum, Tephrosia virginiana, and other organisms with data available.
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
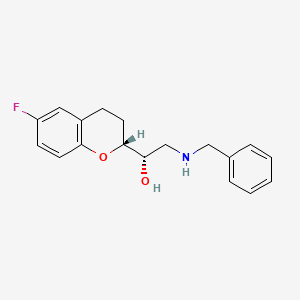
Also known as: 129050-27-7, (1s)-2-(benzylamino)-1-[(2r)-6-fluoro-3,4-dihydro-2h-chromen-2-yl]ethanol, (1s)-2-(benzylamino)-1-((2r)-6-fluoro-3,4-dihydro-2h-chromen-2-yl)ethanol, Ec 603-316-2, Schembl2289517, Dtxsid30648373
Molecular Formula
C18H20FNO2
Molecular Weight
301.4 g/mol
InChI Key
UWHPUMRASBVSQY-FUHWJXTLSA-N
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
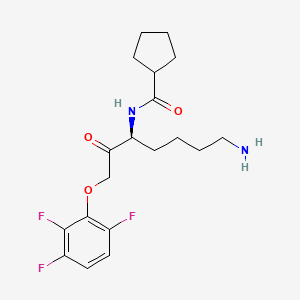
Also known as: Dgn7roz8en, Atuzaginstat [inn], 2211981-76-7, Cyclopentanecarboxamide, n-((1s)-5-amino-1-(2-(2,3,6-trifluorophenoxy)acetyl)pentyl)-, N-((3s)-7-amino-2-oxo-1-(2,3,6- trifluorophenoxy)heptan-3-yl)cyclopentanecarboxamide, Unii-dgn7roz8en
Molecular Formula
C19H25F3N2O3
Molecular Weight
386.4 g/mol
InChI Key
OLIMBXKACVCIMM-HNNXBMFYSA-N
FDA UNII
DGN7ROZ8EN
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
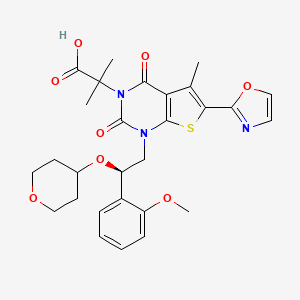
Also known as: Nd-630, 1434635-54-7, Gs-acc, Ndi-010976, Gs-0976, Firsocostat [usan]
Molecular Formula
C28H31N3O8S
Molecular Weight
569.6 g/mol
InChI Key
ZZWWXIBKLBMSCS-FQEVSTJZSA-N
FDA UNII
XE10NJQ95M
Firsocostat is under investigation in clinical trial NCT02781584 (Safety, Tolerability, and Efficacy of Selonsertib, Firsocostat, and Cilofexor in Adults With Nonalcoholic Steatohepatitis (NASH)).
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
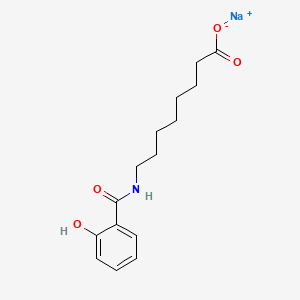
Also known as: 203787-91-1, Snac, Sodium 8-(2-hydroxybenzamido)octanoate, E414, Salcaprozate sodium [usan], Sodium;8-[(2-hydroxybenzoyl)amino]octanoate
Molecular Formula
C15H20NNaO4
Molecular Weight
301.31 g/mol
InChI Key
UOENJXXSKABLJL-UHFFFAOYSA-M
FDA UNII
1YTW0422YU
Salcaprozate Sodium is the sodium salt form of salcaprozate, an oral absorption promoter. Salcaprozate sodium may be used as a delivery agent to promote the oral absorption of certain macromolecules with...
 Upload your Marketing & Sales content on your company Virtual Booth, click HERE
Upload your Marketing & Sales content on your company Virtual Booth, click HERE
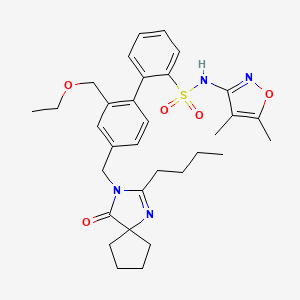
Also known as: 254740-64-2, Sparsentan (re-021), Re-021, Sparsentan [usan], Ps433540, Ps-433540
Molecular Formula
C32H40N4O5S
Molecular Weight
592.8 g/mol
InChI Key
WRFHGDPIDHPWIQ-UHFFFAOYSA-N
FDA UNII
9242RO5URM
Sparsentan has been used in trials studying the treatment of Focal Segmental Glomerulosclerosis. Sparsentan is the first and only dual-acting angiotensin and endothelin receptor antagonist (DARA) in development....