
The new year began with a string of enforcement actions by the US and European regulators against pharmaceutical manufacturers in China. Data integrity and poor facility maintenance were key concerns raised during inspections at some Chinese manufacturing sites, such as Minsheng Group Shaoxing Pharmaceutical Company, Hubei Hongyuan Pharmaceutical Company and Zhejiang Hisoar.
The findings were worrisome. For instance, the French authorities found Minsheng Group Shaoxing Pharmaceutical to be falsifying the origin of the active pharmaceutical ingredient (API) it was selling. Hubei Hongyuan Pharmaceutical’s inspection (by Czech authorities) revealed the manufacturing site and its equipment were “in a devastated state”. And Zhejiang Hisun Pharmaceutical (Taizhou City, Shejiang Province, China) received a warning letter from the US Food and Drug Administration (FDA) due to deficiencies in data management and data integrity.
This week, PharmaCompass delves into what these weaknesses in China’s drug supply chain mean for the global pharmaceutical industry.
How dependent is the world on China?
“When America sneezes the world catches a cold,” – this statement related to the global financial markets nowadays applies more to China, than the US. And it is not restricted to the financial markets. The global dependence on Chinese exports have increased manifolds; and pharmaceuticals is no exception.
Take the case of India. According to a news report, India sources over 70 per cent of its requirement of bulk drugs (or APIs) from Chinese pharma companies. This includes common essential bulk drugs such as Paracetamol, Metformin, Pen-G, 6-APA, Aspirin, Erythromycin Thiocyanate, Sartans, Ofloxacin and Levofloxacin.
At PharmaCompass, we tried to understand the dependence of the global API supply chain on China. In order to get an answer to this puzzling question, our team looked at a report on the “25 most prescribed drugs in the US”. The custom classification codes of a select few drugs were then taken and using global world trade data, a compilation was performed to see how China, India and the United States performed in 2014 with respect to their trade balance (the difference between exports and imports).
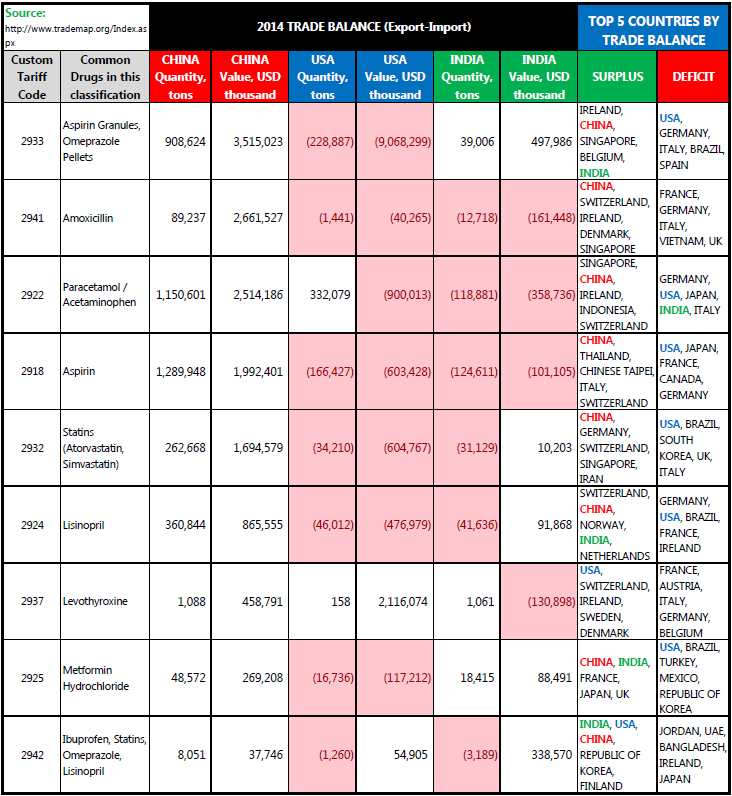
A supply chain disruption is imminent
The table clearly shows the important role China plays in the global marketplace for critical pharmaceutical products. India, a major exporter of finished drug products, is relying on China for majority of its bulk drug imports.
In other cases, the Indian API industry buys the penultimate drug intermediate from China only to “finish” it in India where the compliance standards are considered higher than China. However, in many such instances, the majority of the chemical processing is done in China.
In fact, the world is keeping a close watch on China. The country is the largest producer and user of antibiotics in the world. A comprehensive study done in China has found rivers around densely populated regions severely contaminated with antibiotics. Bacterial resistance rates in hospitals (i.e. superbugs) are found to be directly correlated to the environmental concentrations levels and usage of antibiotics. In fact, antibiotics resistance is a global healthcare challenge.
The threat of antibiotic resistance emanating from China, coupled with GMP compliance concerns at its manufacturing sites are two main issues threatening to disrupt the supply chain from China.
Lack of alternatives to Chinese APIs
In September 2015, PharmaCompass covered Health Canada’s request to quarantine drugs linked to leading Chinese drug maker, Zhejiang Hisun Pharma (Hisun), due to data integrity concerns.
The “interim precautionary measure” was taken “in light of recent findings from a trusted regulatory partner” (read: the FDA) that raised concerns about the reliability of the laboratory data generated at this manufacturing site.
Zhejiang Hisun Pharma qualifies as one of the most prominent API manufacturers in business. Its importance was also demonstrated in the number of exemptions the FDA had to make in order to ensure that there are no drug shortages created as an outcome of the import alert. The US had to exempt fourteen products from its import alert list as alternative suppliers were unavailable and stopping Hisun would have resulted in drug shortages in the United States.
India, for one, is working on a new bulk drug policy to reduce its dependence of China. As part of its ‘Make of India’ initiative, it plans to attract Italian drug makers by doling out attractive incentives.
Our view
There are several countries around the world where the will of the government has led countries to increase their trade surpluses of pharmaceutical products. Countries like Singapore, Ireland and South Korea are a case in the point.
India declared 2015 as the Year of the API and has recommendations which, if implemented effectively, could make India a major supplier of APIs and shift global dependence away from China.
However, given the regulatory action against Indian pharmaceutical companies, coupled with infrastructural bottlenecks in the country, as of today it does not seem like India can be the answer to the world’s problems with Chinese bulk drugs.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : the eyes of truth r always watching u by Gisela Giardino is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







